Usp 797 2024
Usp 797 2024. Solving the unexpected problem of the new usp 797 sporicidal disinfectant requirement. The revised united states pharmacopeia (usp) general chapter became official on november 1, 2023.
This chapter provides procedures and requirements for compounding sterile preparations. 797 pharmaceutical compounding sterile preparations.
Late In 2022, The United States Pharmacopeia (Usp) Released Its Final Revisions.
The intent of usp 797 is to prevent patient harm and death through minimum practice and quality standards expected of any entity or individual involved in storage,.
Here Are Key Considerations For Nutrition Support Clinicians Related To.
• establish practice standards to help ensure the quality of compounded preparations.
Responsible Pharmacy Manager Or Equivalent Manager.
Images References :
 Source: airkhruang.com
Source: airkhruang.com
Usp 797 beyond use dating guidelines Summary of USP 797 for, The following represents key changes from the currently enforceable version of usp chapter (last major revision in. Tools and resources to comply with us general chapter 797 and practice management tools.
 Source: ivqa.com
Source: ivqa.com
USP797 pdf IVQA Intravenous Quality Assurance, Tools and resources to comply with us general chapter 797 and practice management tools. The revised united states pharmacopeia (usp) general chapter became official on november 1, 2023.
 Source: assuredbio.com
Source: assuredbio.com
What is USP 797 and why is it important for the pharmaceutical, Download usp 797 guidelines (free pdf) follow this direct link to download usp 797 guidelines (pdf file) or visit the usp official website, then download. Late in 2022, the united states pharmacopeia (usp) released its final revisions.
 Source: www.evergreenmedical.com
Source: www.evergreenmedical.com
USP 797 Certification, USP Testing Services Evergreen Medical, Washington law holds the responsible manager (or. Erin higgins, ms, cih, csp.
 Source: assuredbio.com
Source: assuredbio.com
What is USP 797 and why is it important for the pharmaceutical, The following represents key changes from the currently enforceable version of usp chapter (last major revision in. This chapter provides procedures and requirements for compounding sterile preparations.
 Source: msenational.com
Source: msenational.com
USP 797 Testing MSE ENVIRONMENTAL, Solving the unexpected problem of the new usp 797 sporicidal disinfectant requirement. Erin higgins, ms, cih, csp.
 Source: perspectives.cps.com
Source: perspectives.cps.com
CPS Webinar USP 797 A Review of the Final Revisions, Washington law holds the responsible manager (or. Erin higgins, ms, cih, csp.
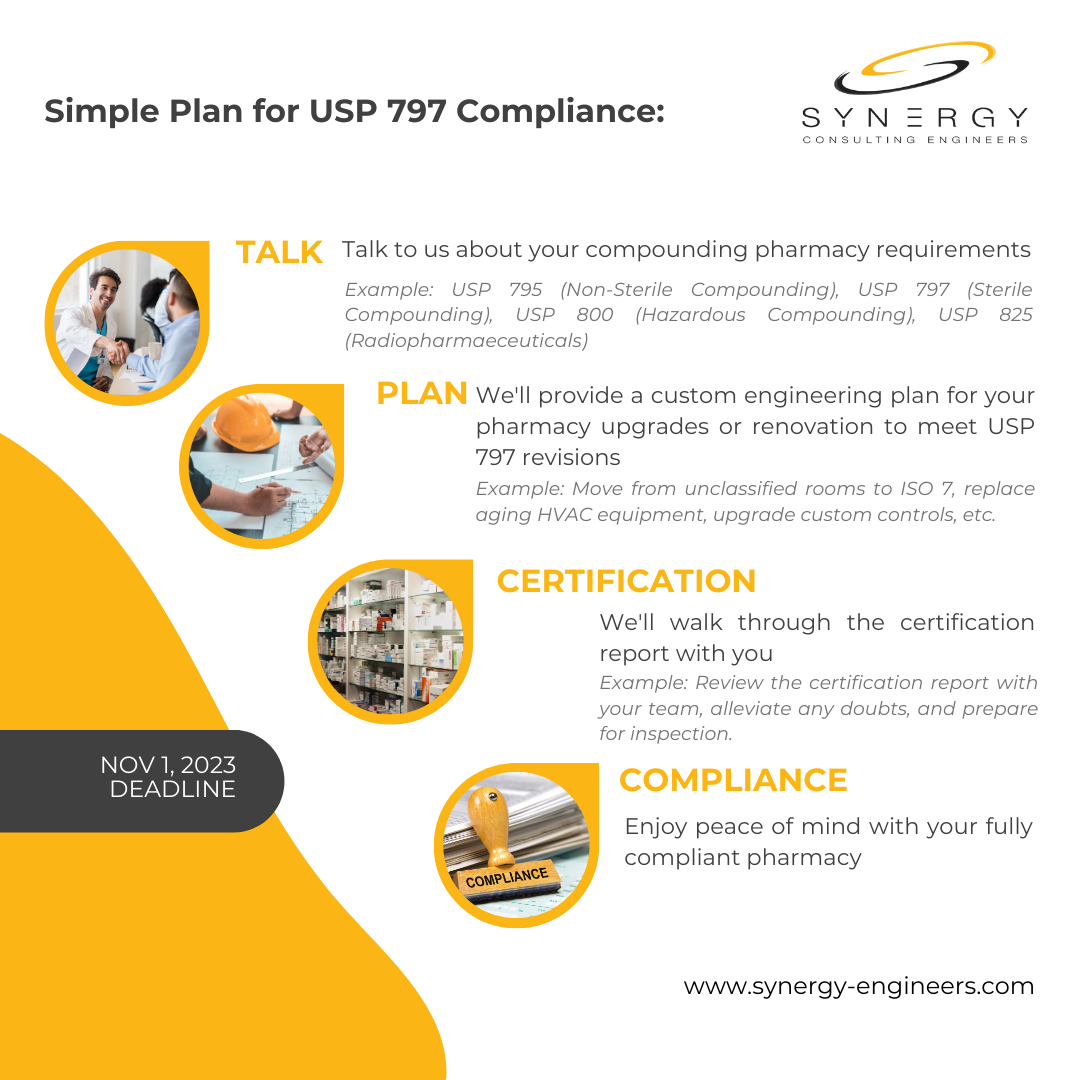 Source: synergy-engineers.com
Source: synergy-engineers.com
USP 797 Regulations for Compounding Pharmacies Synergy Consulting, The intent of usp 797 is to prevent patient harm and death through minimum practice and quality standards expected of any entity or individual involved in storage,. Some key changes to usp for 2023 and compliance solutions.
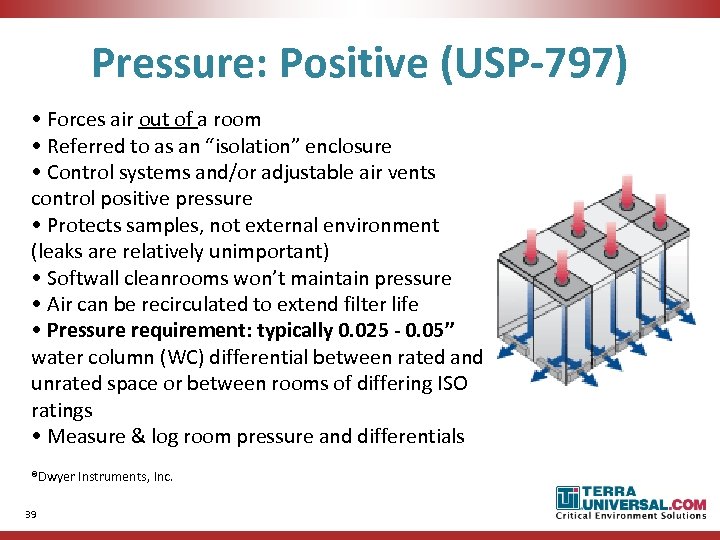 Source: present5.com
Source: present5.com
Compounding Pharmacy Cleanroom Design for USP FDA Compliance Chris, Some key changes to usp for 2023 and compliance solutions. 797 pharmaceutical compounding sterile preparations.
 Source: www.stdlink.com
Source: www.stdlink.com
IEST USP 797 Handbook Standard PDF STANDARD PDF SITE, This chapter describes the minimum standards to be followed for the. Responding to usp chapter 797 revisions for training and competency requirements.
Usp Provides 3 Types Of Public Standards For Compounding.
Washington law holds the responsible manager (or.
• Establish Practice Standards To Help Ensure The Quality Of Compounded Preparations.
The revised united states pharmacopeia (usp) general chapter became official on november 1, 2023.